T cell Technology
Core T-cell Expansion Technology
The core platform technology at the CVPF is the use of artificial antigen presenting cells (aAPCs) for the expansion of T-cells. This includes both proprietary CD3/CD28 bead-based and modified K562 cell-based aAPC technologies to stimulate and expand T-Cells. Both of these matrices – solid beads and self-replicating cells – provide a stimulatory (Signal 1) and co-stimulatory (Signal 2) signal to the T-cells, causing them to “wake up” and divide. A matrix for the cell stimulation is important, because it induces surface receptor cross-linking, which is critical for functional activation. These technologies can result in greater than 100-fold expansion of the T cells. These two approaches allow us to preferentially expand different T cell subsets.
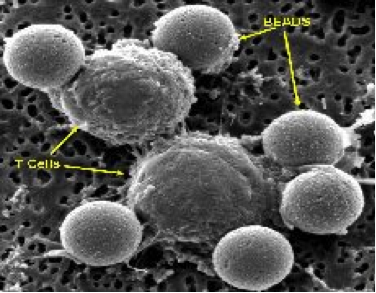
- In the bead-based technology, beads are coated with antibodies that bind to the T cell and activate it. Specifically, the stimulatory signal is an antibody against a protein on the T cell called CD3 (anti-CD3 antibody), and the co-stimulatory signal is an antibody against the CD28 protein (anti-CD28 antibody). Bead-based aAPCs are added to the purified T cell culture at its initiation, and they are removed by magnets (the beads have an iron core) prior to final formulation of the cellular therapy and administration to the patient. Using this approach, as of June 2014, over 1900 T cell infusions have been administered to over 650 patients at the University of Pennsylvania and collaborating centers.
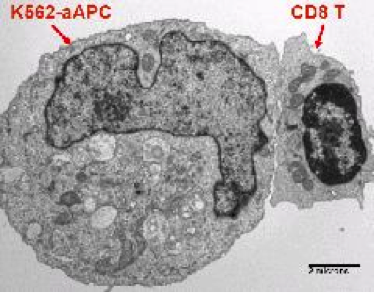
- Dr. Carl June's laboratory has transduced an erythromyeloid cell line, K562, with a library of lentiviral vectors that allows for the customized expression of stimulatory and co-stimulatory molecules, cytokines, and human lymphocyte antigen molecules on their surface to facilitate aAPC-T cell interactions. Up to seven genes of interest at a time have been stably expressed, and this ability to manipulate and express different combinations of genes allows propagation of distinct T cell subsets and phenotypes, including antigen-specific T cells and genetically modified T cells. Lines of interest are transferred to the CVPF for the establishment of Master and Working Cell Banks, which are used to generate aAPCs in support of clinical trials. Investigators, through collaboration with the CVPF, can have access to the use of these reagents for the development of adoptive cellular therapies. The CVPF holds Biologics Master Files (BMFs) describing these cell banks and can provide letters of cross-reference for IND submissions as needed.
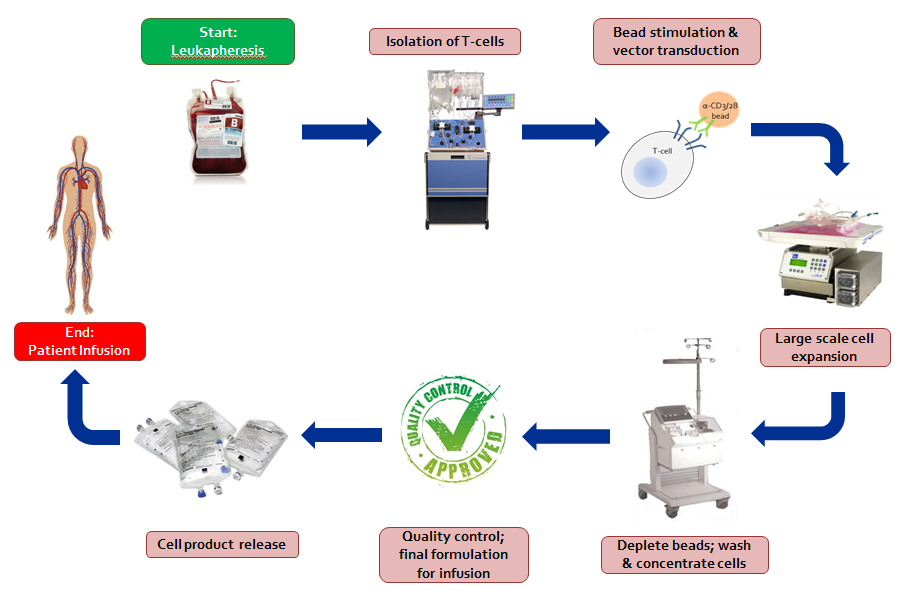
1. Obtain cells from donor and enrich or isolate particular cells of interest
Subjects (patient and/or donor) enrolled in cell-based clinical trials at the Abramson Cancer Center (ACC) of the University of Pennsylvania donate white blood cells by a method called apheresis. Apheresis separates whole blood into its components: red blood cells, white blood cells, platelets, and plasma. A fraction of the circulating white blood cells are collected while all other components are returned to the donor. This is a common and safe procedure performed under medical supervision in the blood banks of the Hospital of the University of Pennsylvania (HUP) or The Children's Hospital of Philadelphia (CHOP). The procedure usually takes two to three hours and the donor may return to normal activities afterwards. The apheresis product is then transported to the CVPF. For clinical trials conducted in collaboration with other medical centers, the apheresis and initial processing may be performed at the remote medical center's collection center and processing laboratory. Particular cells can be enriched or isolated in the CVPF. Depending on the relative percentage of the cells of interest, a positive or negative selection can be performed using monoclonal antibodies directed against particular cell surface markers bound to microscopic magnetic beads. Positive selection targets and isolates the wanted cells while a negative selection targets and isolates the unwanted cells by means of magnetic separation.
The product is loaded into the disposable kit and is pumped through the tubing and wash circuit of the Cell Saver® 5 as the freezing media is removed and replaced with wash buffer. The final washed product is then concentrated and re-suspended for further manipulation steps, as below.
2. Stimulate cells to grow
White blood cells can be stimulated to grow in the laboratory by a variety of methods. The cells are cultured in nutrient-rich media, but need a source of stimulation in order to divide and grow. In our facility, this is provided by binding monoclonal antibodies directed against cell surface receptors to microscopic magnetic beads as artificial stimulator or antigen presenting cells (see Core T-cell Expansion Technology). Second generation artificial antigen presenting cells (aAPCs) engineered to express a variety of combinations of stimulator molecules are currently under development and validation. The mixture of cells, growth media, and monoclonal antibodies immobilized on beads is added to gas permeable plastic bags and stored in humidified incubators at 37 °C. The cells begin to divide, and after a few days, clumps of cells attached to the antibody-coated beads can be seen growing in the bags
The brownish-red colored beads are washed in culture media. A magnet is then applied so that the beads will adhere to the side of the tube during removal of the wash buffer, thereby minimizing bead loss during this process
3. Insert potentially therapeutic genes into cells
Immune cells and stem cells can be redirected, given anti-tumor or anti-viral properties, or given enhanced survival properties via the transfer of therapeutic genes. These genes are inserted first into a carrier to serve as a delivery vehicle to the cell termed a “vector.” The vector, added to an activated cell population, enters the cell and releases its payload, the gene. The cell then takes up the gene and expresses the product of the gene.
4. Large scale cell expansion
Standard cell culture flasks are not well suited for large scale culture of human cells for clinical trials. The number of cells that can be grown in these flasks is limited, and to reach the therapeutic numbers of cells desired would present space and labor requirements that are impractical. In addition, these flasks must be opened to sample and maintain the cells during culture, and although this is done in a cabinet with filtered air, the potential for contamination with bacteria, mold, or fungus exists. For these and other reasons, the CVPF cultures cells in closed gas-permeable plastic bags. Tubing leads on the bags and a variety of connecting devices allow the cells to be grown in a closed system with minimal risk of contamination. In addition, these bags can contain several billion cells each and thus reduce space requirements and the "hands-on" time needed to maintain the culture.
This video depicts tubing on the closed system cell culture bag being clamped and heat-sealed, the cell bag gently rocked to re-suspend cells and beads in the media that was added, and the culture bag placed into an incubator for cell expansion.
New media is introduced when the cells need a fresh source of nutrients and protein. The cell culture bag is connected to a fresh media bag that is elevated above the culture bag, enabling the media to flow down to the culture by gravity. The amount of media is monitored with a scale; when enough is added, clamps are closed and tubes are re-sealed.
5. Remove beads and wash cells
As mentioned above, the cells are stimulated using antibody-coated beads, and to grow therapeutic numbers of cells, large volumes of culture media are required. Before infusing the cells into a patient, the beads must be removed, the culture media washed off, and the cells concentrated into a small volume. Maintaining a closed system throughout, the cell culture is passed over very powerful magnets that retain the beads while the cells flow through. The bead-free cell culture is then loaded into a specialized centrifuge that washes and separates the cells from the growth media. At this point, the cells are re-suspended in an infusible solution or cryopreserved for future use. Cells that are infused fresh or cryopreserved for later infusion must meet all quality control and release criteria before release from the CVPF.
Please click on the picture above for a larger picture of the magnetic separator that is used to remove the beads from the cell culture after expansion.
6. Quality Control (QC)
To be infused, the cells must be certified to be free of contaminating agents and to meet certain requirements for cell purity or phenotype, identity and potency. This is the responsibility of the Quality Control component of the CVPF. Testing for contaminating agents such as bacteria, fungi, mycoplasma, bacterial endotoxins, and HIV p24 (if applicable) can be performed in our laboratory or on contract with clinical reference laboratories. Cell viability and phenotyping are performed in our laboratory. The results from these assays are recorded on Certificate of Analysis forms prior to the release of cells from the CVPF and accompany the cells to the infusion site. An independent Quality Assurance officer oversees the Quality Control process