Recent Publication
The CVPF is excited to announce an article recently published in The New England Journal of Medicine. To view the full research article online, please click here
ORIGINAL ARTICLE | BRIEF REPORT
Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma
Alfred L. Garfall, M.D., Marcela V. Maus, M.D., Ph.D., Wei-Ting Hwang, Ph.D., Simon F. Lacey, Ph.D., Yolanda D. Mahnke, Ph.D., J. Joseph Melenhorst, Ph.D., Zhaohui Zheng, M.S., Dan T. Vogl, M.D., Adam D. Cohen, M.D., Brendan M. Weiss, M.D., Karen Dengel, R.N., B.S.N., Naseem D.S. Kerr, M.P.H., Adam Bagg, M.D., Bruce L. Levine, Ph.D., Carl H. June, M.D., and Edward A. Stadtmauer, M.D.
A patient with refractory multiple myeloma received an infusion of CTL019 cells, a cellular therapy consisting of autologous T cells transduced with an anti-CD19 chimeric antigen receptor, after myeloablative chemotherapy (melphalan, 140 mg per square meter of body-surface area) and autologous stem-cell transplantation. Four years earlier, autologous transplantation with a higher melphalan dose (200 mg per square meter) had induced only a partial, transient response. Autologous transplantation followed by treatment with CTL019 cells led to a complete response with no evidence of progression and no measurable serum or urine monoclonal protein at the most recent evaluation, 12 months after treatment. This response was achieved despite the absence of CD19 expression in 99.95% of the patient’s neoplastic plasma cells. (Funded by Novartis and others; ClinicalTrials.gov number, NCT02135406.)
The CVPF is excited to announce an article recently published in Science Translational Medicine. To view the full research article online, please click here.

RESEARCH ARTICLE | IMMUNOTHERAPY
Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia
Immunotherapy is one of the most promising avenues of cancer therapy, with the potential to induce sustained remissions in patients with refractory disease. Studies with chimeric antigen receptor (CAR)–modified T cells have paved the way in patients with relapsed and refractory chronic lymphocytic leukemia. Porter et al. now report the mature results from their initial CAR T cell trial. CAR T cell persistence correlated with clinical responses, and these cells were functional up to 4 years after treatment. No patient who achieved complete remission relapsed, and no minimal residual disease was detected, suggesting that in a subset of patients, CAR T cells may drive disease eradication.
Patients with multiply relapsed or refractory chronic lymphocytic leukemia (CLL) have a poor prognosis. Chimeric antigen receptor (CAR)–modified T cells targeting CD19 have the potential to improve on the low complete response rates with conventional therapies by inducing sustained remissions in patients with refractory B cell malignancies. We previously reported preliminary results on three patients with refractory CLL. We report the mature results from our initial trial using CAR-modified T cells to treat 14 patients with relapsed and refractory CLL. Autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector were infused into patients with relapsed/refractory CLL at doses of 0.14 × 108 to 11 × 108 CTL019 cells (median, 1.6 × 108 cells). Patients were monitored for toxicity, response, expansion, and persistence of circulating CTL019 T cells. The overall response rate in these heavily pretreated CLL patients was 8 of 14 (57%), with 4 complete remissions (CR) and 4 partial remissions (PR). The in vivo expansion of the CAR T cells correlated with clinical responses, and the CAR T cells persisted and remained functional beyond 4 years in the first two patients achieving CR. No patient in CR has relapsed. All responding patients developed B cell aplasia and experienced cytokine release syndrome, coincident with T cell proliferation. Minimal residual disease was not detectable in patients who achieved CR, suggesting that disease eradication may be possible in some patients with advanced CLL.
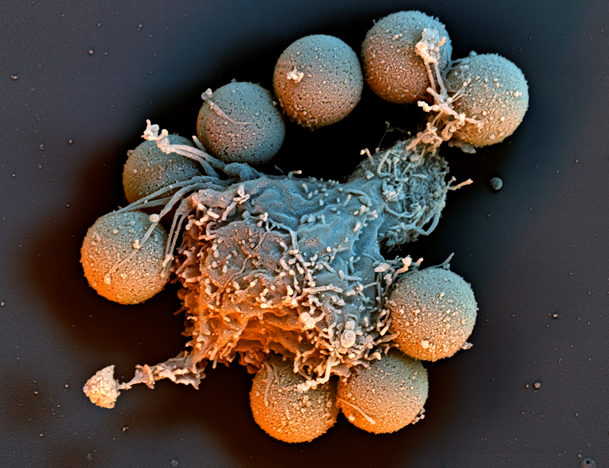
A nanoflight View of Immune Cells Engineered to Fight Cancer
Sample Preparation by Ray Meade,
Electron Microscopy Resource Lab, Perelman School of Medicine
nanoflight video by © Scientific Photography – Stefan Diller, Germany